Change Page: 1234 > | Showing page 1 of 4, messages 1 to 20 of 71 - powered by ASPPlayground.NET Forum Trial Version
Reports tied to this Journal
Author
|
Message

|
 Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 12:08 AM
Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 12:08 AM
( permalink)
Culturing Journal DataSheet
This first post should be updated regularly to include new information as events take place or changes are made to your system General Species: Parvocalanus crassirostris Species description: Calanoid copepod Culture source (link if possible ): Thoughtful gift from Dr. Andrew Rhyne If algae, CCMP # (Optional ): http://ccmp.bigelow.edu/ Culture Establishment Date: 1/8/2013 Continuation Date: 4/8/2013 Culturing Vessel Details Salinity: 1.020 - 1.026 Temperature: 72-74 F pH: Not measured
Vessel description: Various, but mostly 2L borosilicate media jars, and 1000 mL Erlenmeyer flasks Lighting description: Ambient fish room light. Lighting cycle: Typically 18 hours on / 8 hours off. Aeration description: Very light. Rigid airline set near the bottom, and about 1-2 bubbles per second. Methodologies Split methodology: Various. See notes in Journal. Culture medium description: New, aged artificial salt water. Fed live phytoplankton, usually a monodiet of Isochrysis, very sparingly and often. See notes in Journal. Cell count: Varies as population of nauplii vs. adults varies over time. Reference links: http://bit.ly/Xxlkbe http://www.sciencedirect..../pii/S0044848603001613 Additional Information (No Pictures or Videos in the Section Please) Notes: You will be required to provide photographic evidence and as much detail as possible about your project in this thread.
If your thread does not contain detailed enough photos and information the MBI Council will not be able to approve your reports.
<message edited by JimWelsh on Tuesday, May 14, 2013 11:54 AM>
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 12:16 AM
Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 12:16 AM
( permalink)
Here is a composite image I assembled from about 6 different images of the same individual copepod I took today with the microscope/camera combo I have access to at work. I think this is an adult female. It was one of the largest individuals I could find in the sample I was working with. 
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 12:26 AM
Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 12:26 AM
( permalink)
I used the software at the lab to measure the smallest nauplius I could find (I think it may be an N1 nauplius [EDIT: After more reading, I'm now almost certain that it is an N2 nauplius, and not an N1], but I'm not sure) and also the largest adult I could find. The nauplius is 45 microns wide, and 82.6 microns long. The adult has a prosome length of appx. 400 microns, a Total Length of appx. 530 microns, and a width of appx. 200 microns. Here are the images: 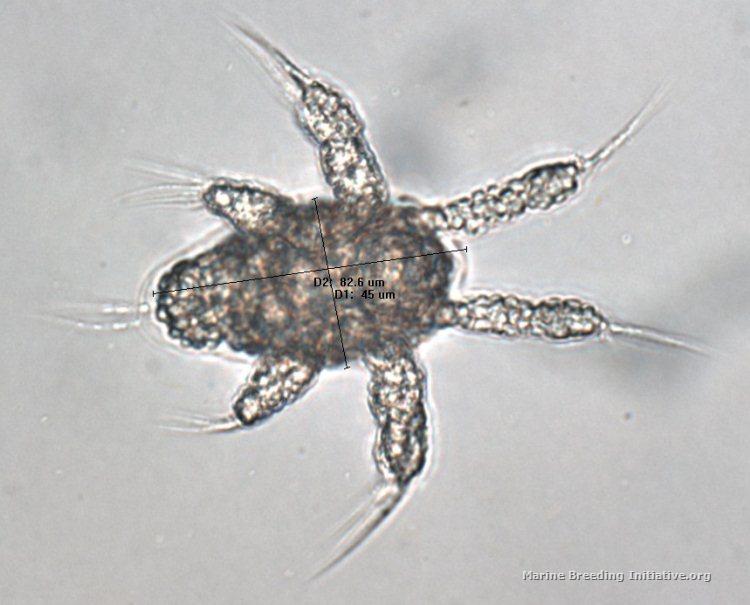 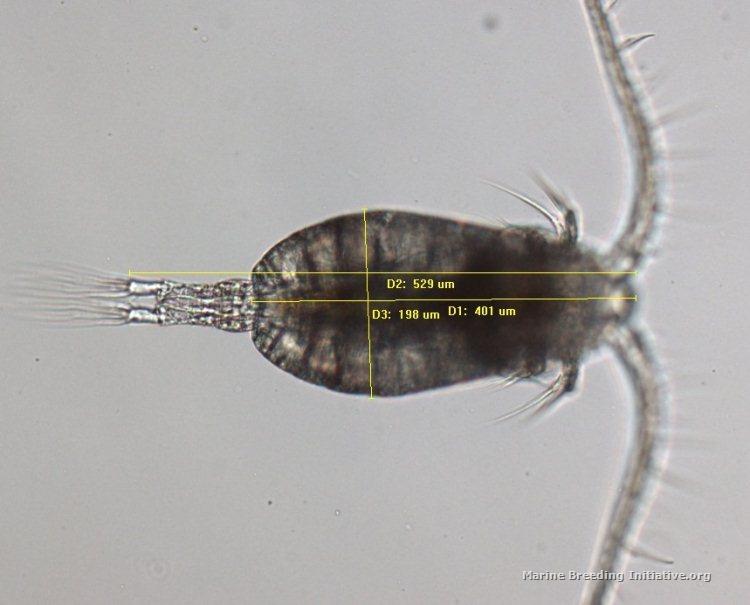 EDIT: Note that the nauplius was photographed at 400x, while the adult was photographed at 100x.
<message edited by JimWelsh on Wednesday, January 23, 2013 3:37 AM>
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 12:47 AM
Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 12:47 AM
( permalink)
Here is a combined image of the adult and the nauplius at the same magnification factor. 
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 2:11 AM
Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 2:11 AM
( permalink)
Great pics!
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 7:41 AM
Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 7:41 AM
( permalink)
The N1 is tiny and more square, lacking the bud at the posterior. That bud becomes hooked shaped shortly in N3-N6. We're getting ready go from egg to adult to egg in a control study to look at duration and size distribution. I can get you that information.
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 10:48 AM
Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 10:48 AM
( permalink)
Great info Jim. Its funny the timing of this in contrast to the info I was looking at for the gobies. I'll scope my cultures to see what I have this weekend.
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 4:58 PM
Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 4:58 PM
( permalink)
Great photos, Jim!
--Andy, the bucket man. "Not to know the mandolin is to argue oneself unknown...." --Clara Lanza, 1886
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 5:03 PM
Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 5:03 PM
( permalink)
I have found some good information in "Handbook for the Cultivation of two Hawaiian Paracalanid Copepods" by Kyle VanderLugt and Petra H. Lenz. The PDF can be found here: http://bit.ly/Xxlkbe (please forgive them for getting Harpactioids and Calanoids mixed up in the caption of Figure 4 on Page 5). The good stuff starts on page 12, where Section 4 on "Copepod Cultivation" starts. There is a primer on phytoplankton production and the use of a hemacytometer to measure algal density. The algal density part would seem to be an important part of maintaining healthy Parvocalanus cultures that is, in my interactions with other hobbyists, almost entirely overlooked. I think that having a reasonable idea of the density of your algal cultures, and thus, how much algae to feed the copepod cultures, may be one key to success with Parvocalanus. The density recommended in this Handbook ranges from as low as 5,000 cells per ml up to 100,000 cells per ml, depending on copepod density and whether high nauplii production is desired. They also note that while it is important not to let the copepods starve, problems can arise if the algal density gets too high. Isochrysis cultures near the end of the exponential phase or approaching the plateau phase of growth may contain anywhere from as little as 1 million cells per ml up to perhaps 15 million cells per ml, depending on many variables in the phytoplankton culture protocol used. In order to know how much algae to add to your copepod culture, you have to have some idea of how dense it is in the first place. Florida Aqua Farms sells simple Secchi algal density measuring sticks ( http://florida-aqua-farms...ae-density-measurer/), together with a simple chart for estimating algal density for various species of microalgae. If I am able to come up with a good approximation of how dense my algae culture is, then I will be able to intelligently add algae to my copepod culture to ensure that they are being fed enough, but not being overfed. Terms such as "lightly tinted" or "weak tea", or other such subjective descriptions of what a given algal density is supposed to look like have always bothered me. I did bring some of my Isochrysis into the laboratory where I work yesterday, and used the hemacytometer there to do a cell count. The result were interesting. While the Secchi stick / chart method of estimating the density indicated I should have had appx. 15 million cells per ml (around 3.5 cm on the Secchi stick), the hemacytometer count returned a result closer to 6 million cells per ml. Granted, it was the first time I used a hemacytometer, and talking to the microbiologists at the lab, they weren't surprised at all by the discrepancy, and they said that considering the potential for sampling errors and numerous other variables, they considered my count to be relatively close to the Secchi reading. The Secchi stick method is subject to variables, too, such as the amount of light in the environment when taking the reading. I plan to do some more cell counting soon, though, to see if I can get a better sense of what my actual algal densities are. I could keep going on, but will stop now, before this post turns into a book!
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 6:52 PM
Re:Culture Journal, Species: Parvocalanus crassirostris
Wednesday, January 23, 2013 6:52 PM
( permalink)
i have no problem with a book… keep going, please.
check out Kathy's Clowns, llc website: http://kathysclowns.com Captive bred clownfish and more (Wholesale to the trade.)
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Thursday, January 24, 2013 2:15 AM
Re:Culture Journal, Species: Parvocalanus crassirostris
Thursday, January 24, 2013 2:15 AM
( permalink)
Let the book continue: When it comes to quantifying algal density, cells per milliliter is an obvious commonsense measurement. The scientific literature, though, often refers to algal density in terms of units of the carbon contained within the algae per given volume, expressed as micrograms of carbon per liter, for example. This allows for a consistent delivery of algal nutrition regardless of which species of algae, or mixture of algae, is being used, as long as you know the amount of carbon per cell of the various algae species being used as food sources. With this approach, you establish a target level of carbon density, usually expressed as micrograms of carbon per liter in the culture media, and then determine the appropriate cell density based on the carbon content per cell of the algae being used, which is usually expressed in terms of picograms of carbon per cell. This target level is frequently expressed in terms of a "saturation level" of carbon for the organism in question. Let me give a practical example. In the paper, "Egg production, egg hatching success and population increase of the tropical paracalanid copepod, Bestiolina similis (Calanoida: Paracalanidae) fed different microalgal diets" by Caymus, et. al., 2009, they feed the Bestiolina a "saturation level" of 1500 micrograms of Carbon per liter, which is "known to saturate copepod feeding", according to another paper, "Bioenergetics of the planktonic copepod Acartia tonsa: relation between feeding, egg production and respiration, and composition of specific dynamic action", by Kiørboe, et. al., 1985. I haven't yet found a good source for determining the carbon content per cell of various algae species, aside from the data that can be seen in Table 1 in the paper, "The potential of tropical paracalanid copepods as live feeds in aquaculture" by McKinnon, et. al., 2003, and I have yet to determine the source of the data in that table. Nonetheless, according to that table, Isochrysis has 17.8 picograms of Carbon per cell. Remember that we are trying to translate a target value of micrograms of Carbon per liter into a number of cells of algae per milliliter. That implies a factor of 1,000 (liter => milliliter). Remember also that we are translating micrograms of carbon per liter into picograms of carbon per cell. That implies a factor of 1,000,000 (micrograms => picograms). The 1,000,000 divided by the 1,000 equals a factor of 1,000 (picograms per cell => micrograms per liter). That said, assuming a target Carbon concentration of 1,500 micrograms of Carbon per liter, and a Carbon concentration of 17.8 picograms of carbon per cell for Isochrysis, that means that we will need 1,500 / 17.8 * 1,000 = appx. 84,270 cells per milliliter of Isochrysis to achieve our target density. There are various other references to algal density for culturing Paracalanid copepods (many studies have involved Bestiolina rather than Parvocalanus, but they are closely related enough that IMHO, Bestiolina data is quite relevant to Parvocalanus culture). Various scientific papers about either Parvocalanus and/or Bestiolina (e.g., "Intensive Cultivation of the Calanoid Copepod Bestiolina similis" VanderLugt 2005) tend to indicate that the best results, in terms of survival rates and egg and nauplius production, can be achieved with algal densities equivalent to somewhere between 10,000 and 100,000 cells per milliliter for Isochrysis. The value arrived at in the previous paragraph is right in line with these guidelines.
<message edited by JimWelsh on Tuesday, June 25, 2013 6:18 PM>
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Thursday, January 24, 2013 12:48 PM
Re:Culture Journal, Species: Parvocalanus crassirostris
Thursday, January 24, 2013 12:48 PM
( permalink)
phytoplankton cells are not a set size though, they change slightly with growing conditions, thus the carbon contentcan be different per cell. This is why we don't offer cell counts on our Instant Algae products, it depends on what time of year it is.
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Thursday, January 24, 2013 2:04 PM
Re:Culture Journal, Species: Parvocalanus crassirostris
Thursday, January 24, 2013 2:04 PM
( permalink)
I'm not convinced that the Isochrysis growing in a closet in my basement at a constant temperature is really affected by the day length outside. Still, assuming that there is a slight variation, if one is using a turbidity / light attenuation method of estimating cell density, then it would take fewer cells of a larger size, or more cells of a smaller size to attenuate the light sufficiently to achieve a given reading on my Secchi stick. In other words, the carbon content for a given Secchi depth should even out, even if the cell count varies, wouldn't you think? What I am attempting to do in this journal is to document my thought process as I try to apply the information contained in carefully controlled scientific studies to my hobbyist implentation in a practical way. If I get close enough to maintain my Parvocalanus cultures instead of killing them (as I have done multiple times in the past), then I will have achieved my goal. Now that I have an idea of the target concentration in my copepod culture, and also an idea of how dense my Isochrysis culture is, I can proceed to estimate how much algae I need to add to my copepod culture water to establish that concentration. For instance, if my Isochrysis has a density of 6 million cells per ml, and I want to end up with around 84,000 cells per ml in my copepod culture water, then I need to add 1,000 * 84,000 / 6,000,000 = 14 ml of algae to each liter of culture water. What does that look like? A lot like water without any algae in it, frankly! Here is a picture two flasks, one with and one without 14 ml of Isochrysis that I estimate to be 6,000,000 cells per ml:  It reminds me a lot of trying to read a phosphate test at the very low end. That's some pretty weak tea, and it's also VERY lightly tinted.
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Thursday, January 24, 2013 2:09 PM
Re:Culture Journal, Species: Parvocalanus crassirostris
Thursday, January 24, 2013 2:09 PM
( permalink)
Temp is not just what effects it, nor just photo-period. Lighting type (spectrum, pulse rate, etc), salinity, nutrients, etc all play a roll as well. Of course the sun won't effect it in your case, but I was not suggesting that. Sometimes I wonder why I even post here with comments like that, seriously.
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Thursday, January 24, 2013 2:41 PM
Re:Culture Journal, Species: Parvocalanus crassirostris
Thursday, January 24, 2013 2:41 PM
( permalink)
I am trying to understand how significant the effect you are describing is to the practical application of my breeding operation. Even if there is some minor variation in the cell size due to uncontrolled variables, I would expect that variation to be slight in comparison to other variables I'm dealing with, such as the accuracy with which I can estimate the cell count in my phyto culture. I'm just trying to get somewhere close to the right density. When published accounts of what the "right density" is ranges from 10,000 to 100,000 cells per ml, then there is an order of magnitude or more between "too little" and "too much". I'm just trying to land somewhere in that range. Unless I misunderstand, and the effect you are describing can cause a really significant change in the carbon content per cell (greater than the likely measurement error of my eyeballing it with a Secchi stick, for instance) then I'm just not sure this is an effect I need to factor in.
I do appreciate your comments and input. I'm just trying to process your comment in the context of what my goal is here. I'm sorry if my flip response questioning the significance of the effect to my process offended you.
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Thursday, January 24, 2013 3:47 PM
Re:Culture Journal, Species: Parvocalanus crassirostris
Thursday, January 24, 2013 3:47 PM
( permalink)
Obviously you still cannot grasp what I did not like as you are repeating it yet again  . Carry on, I have no desire to continue with this, at all.
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Thursday, January 24, 2013 4:10 PM
Re:Culture Journal, Species: Parvocalanus crassirostris
Thursday, January 24, 2013 4:10 PM
( permalink)
Let me try a different approach, please.
Please help me understand how great this variation in cell size, and thus, the carbon content is, Gresham. I'd like to learn more about this. Do I need to learn how to estimate the cell size / cell carbon content in addition to estimating cell density? If so, do you have any suggestions about how I can go about doing that?
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Thursday, January 24, 2013 6:32 PM
Re:Culture Journal, Species: Parvocalanus crassirostris
Thursday, January 24, 2013 6:32 PM
( permalink)
Stunning pictures Jim Welsh
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Friday, January 25, 2013 3:27 AM
Re:Culture Journal, Species: Parvocalanus crassirostris
Friday, January 25, 2013 3:27 AM
( permalink)
Thank you, Peerless.
The scientific paper "Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton" by Menden-Deuer and Lessard, 2000, in Table 4, gives formulae for calculating Carbon content per cell from cell volume for various types of planktonic organisms. Using the formulae in that table, and calculating the volume of Isochrysis as a prolate spheroid ("Biovolume Calculation for Pelagic and Benthic Microalgae", Hillebrand, et. al., 1999), with height (h) and diameter (d) ranging from h= 6.8 to 7.2 um and d = 3.8 to 4.2 um ("A comparative study of the inhibitory effect of diatoms on the reproductive biology of the copepod Ternora stylifera", Ianora, et. al., 1995), gives a minimum pg of Carbon per cell of 16.5, a maximum of 20.6, and a median of 18.5, which is a range of +/- 11% from the median. This variation is far less than the accuracy of my ability to estimate cell density using a Secchi stick, IMHO.
Moving on....
Having established an initial density of algae appropriate for feeding Parvocalanus, the next question is how quickly the algae is being cleared, and thus, how much additional algae needs to be added over time to maintain the desired density. While there are studies about clearing rates, it strikes me that one could simply monitor the turbidity / light attenuation of the culture water to determine how much additional algae needs to be added to return the density to the desired level. Clearly, one could use a hemocytometer regularly to perform cell counts, but this is a time consuming and tedious endeavor (albeit a robust one). If a quicker and easier method of estimating the cell density could be developed that was sufficiently accurate and precise, this would be desirable.
Setting aside the algal density question for a moment, another potential area of concern is the density of the Parvocalanus copepods themselves in the culture. Studies have shown that excessive density of adult copepods can have an adverse effect on nauplii production, e.g., "Management of nauplius production in the paracalanid, Bestiolina similis (Crustacea: Copepoda): Effects of stocking densities and culture dilution", VanderLugt and Lenz, 2008. In short, assuming that you succeed in producing a large number of copepods in your culture, and a large number of them survive to adulthood, it is very likely that the increased density will result in a significant decrease in egg production, and possibly cause a sudden crash of the copepod culture, once the adult population dies off due to the limited natural lifespan. In other words, if you are successful at culturing these copepods, it is important to regularly split, harvest, dilute or otherwise manage the adult density in order to sustain a relatively high nauplii production rate. More on this to follow....
|
|
|
 Re:Culture Journal, Species: Parvocalanus crassirostris
Saturday, January 26, 2013 12:24 AM
Re:Culture Journal, Species: Parvocalanus crassirostris
Saturday, January 26, 2013 12:24 AM
( permalink)
I took another sample of my dense, dark Isochrysis that measures 3.5 cm on my Secchi stick (appx 15 million cells per ml, according to my FAF chart) into the lab today, and did a more careful and scientific hemocytometer measurement of the density. First, I dealt with the motile nature of the cells by doing a 2:1 dilution with 70% denatured ethanol, thus killing the cells, making them settle more quickly, and much easier to count. Second, I did two measurements, and averaged the two results. In the first measurement, I counted the cells in all 25 squares of the hemocytometer grid. The count was 665. So, 665 * 10,000 * 2 (dilution factor) = 13,300,000. In the second count, I counted the 4 corners plus the middle square, and multiplied the result by 5. The count of the 5 squares was 166. So, 166 * 5 * 10,000 * 2 = 16,600,000. The average of 13,300,000 and 16,600,000 = 14,950,000, which is pretty darn close to 15,000,000!
I also took some pictures of my Isochrysis, and used the measuring software to get measurements of about 20 randomly selected cells. The average width was 4.36 microns, with a minimum of 3.55 and a maximum of 4.93. The average length was 6.37 microns, with a minimum of 5.72 and a maximum of 6.87. Based on these values, the average cell volume using the formula for a prolate spheroid is 4.36 * 4.36 * 6.37 * pi / 6 = 63.0 cubic microns. Plugging that value into the formula described in my previous post by Menden-Deuer and Lessard 2000, the picograms of Carbon per cell = 19.7. Using this value, the target cell density to achieve the "saturation density" of 1500 micrograms of Carbon per liter = 1500 / 19.7 * 1000 = appx. 76,000 cells per ml. In other words, I will need to add 1000 * 76,000 / 15,000,000 = appx. 5.1 ml of my dense Isochyrsis to each liter of copepod culture media to achieve the target "saturation density".
I brought the hemocytometer home with me for the weekend, and hope to be able to work with such dilutions and confirm the target cell counts. At the same time, I am going to try to work up a colorimetric method for quickly and easily estimating the algal density of the copepod culture water, but more on that later.
By the way, I've been observing my various cultures of Parvocalanus over the last several days. All of them are doing well, and all of them have numerous nauplii visible, as well as numerous adults. A general definite trend is that the cultures that are more dense in terms of adults tend to be less dense in terms of nauplii, and vice versa. The general concept that an excessive adult density suppresses egg/nauplii production seems to have merit.
|
|
|
|